SmartX – Providing Process Control Solutions

The pharmaceutical industry is evolving as more and more companies begin to embrace Industry 4.0 technologies in an effort to modify approaches to drug development and manufacturing. SmartX process automation is InnoGlobal Technology’s (formerly Innopharma Technology) Industry 4.0 platform, designed to accelerate process development and to automate process control in the Pharmaceutical and related manufacturing industries. By delivering powerful data-driven process insights and connected manufacturing, SmartX ensures better quality, reliability, and efficacy, as well as efficient regulatory compliance. Deeper process understanding paves the way for greater process-specific control and optimization solutions. This blog will take a closer look at one example of how the SmartX system can lend itself to creating both a flexible and novel control solution for a commonly experienced fluid bed granulation problem.
The Problem
Nozzle fouling issues are extremely disruptive to the granulation process. Blockages can arise following evaporation of liquid inside the spray nozzle during a process, leading to deterioration of atomization during the spraying phase. In addition, wetting and adhesion of material around the nozzle tip can cause build-up. This can interfere with the normal spray pattern, causing a dripping effect which leads to the formation of large agglomerates, which can also affect fluidization within the product container.
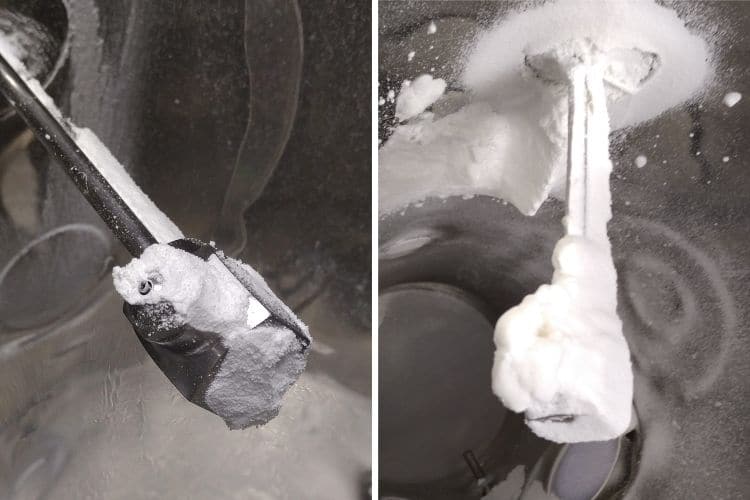
During a recent body of work involving SmartX for a number of experimental granulation batches at our in-house application lab, an intermittent problem was observed impacting the amount of oversized end-product for several of these batches. Occurrence of this oversized material was linked to a build-up of product adhering to the nozzle tip during the spraying phase. It is essential to tightly control the amount of these oversized agglomerates to ensure that effective drying can take place, any impact on downstream processing is avoided and of course to maintain good yield efficiency.
The Solution
To remedy this nozzle fouling issue, an airflow sensor was installed on the atomizing airflow delivery line to monitor the resulting atomizing airflow. Atomization is controlled by pressure regulation and so monitoring the resulting atomizing airflow rate was considered to be a meaningful way to identify any downstream obstruction to the atomizing airline. Once a nozzle fouling signature was successfully detected from the sensor data i.e. an observed drop in atomizing airflow rate, it was possible to use the sensor data within the SmartX controller to implement an automated controlled response to address the onset of nozzle fouling.
Firstly, it was important to explore the viability of this solution under manual control conditions, to determine appropriate parameter control values, and ultimately confirm this particular approach could deliver the intended result. When the nozzle fouling signature was detected in the output sensor data, the atomizing spray rate was manually reduced while also simultaneously increasing atomizing pressure to purge the nozzle tip for a time. Once purging had been completed the atomizing airflow rate was re-checked to ensure the fouling issue had been corrected.
Confident the approach had merit, the SmartX control logic was updated to incorporate the airline sensor measurement data. This meant that when a nozzle fouling ‘signature’ was detected by SmartX, the automated controller would initiate a response similar to the manual intervention outlined above, however this time it would be dynamic and autonomous, continuously reacting to real-time process conditions inside the product container. The controller executes a response, proportionate to the degree of deviation occurring in normal atomizing air value, adjusting key parameters; spray rate, and atomizing pressure, until normal process conditions return.
In addition, to implementing dynamic control, the controller was configured to monitor the airline as a preventative measure. Periodic high-pressure purging of the nozzle tip during the spraying phase was executed, once conditions inside the product container exceeded a certain moisture limit, considered to be the most vulnerable time of the process due to the high moisture content present. The automated controller increasing the atomizing pressure from the baseline value for a defined period of time, and repeated at frequent intervals during this ‘high moisture’ and ‘high risk’ stage of the process.
This automated control solution is just one example of the flexibility and potential offered by SmartX in delivering greater process optimization and control. This technology makes it possible for the Process Development Engineer to conjure up new and creative solutions to remedy process development problems, delivering real value-added solutions that can really make a difference.
At InnoGlobal Technology (formerly Innopharma Technology), we’re about pushing industry forward with trailblazing innovation. For more exciting news and the latest developments on our SmartX Platform, follow us on LinkedIn and Twitter.

